Platinum is a silver-looking, dense, remarkably stable, and extremely rare metal. First discovered for western science by conquistadors, it famously got its name from platino or “little silver” for its obvious resemblance to silver in physical appearance. At the time, however, they did not think much of it and considered it pretty useless, an impurity in gold, as they could not melt it.
It was only after it was brought to Europe in the 18th century that it started to be investigated for its properties. Eventually, as time went on and technology developed, platinum became recognized for its exceptional properties and gained on its value. One of the things that keep the price of platinum high today is the fact that it is rare to find in nature, just like other platinum group metals (platinum, palladium, rhodium, ruthenium, iridium, osmium). In today’s world, this means that platinum is used either for luxury goods or when some functionality is desperately needed. It also means that when platinum is needed for its unique surface properties, bulk metal is rarely used, and is replaced whenever possible with thin platinum coatings on less precious substrates.
In this article, I will list some of the most common use cases of platinum coatings:
1. Luxury
Award rankings where platinum is placed higher than gold speak to the perceived exclusivity and luxury of the metal, despite its silver-like appearance. In addition to its rarity, platinum is exceptionally durable and scratch-resistant. With its bright metallic shine which does not tarnish, it is the preferred metal to use for the decoration of high-value luxury goods.

2. Medical devices and implants
Platinum is inert and does not react with air, water, bases, and most acids. Unlike many other metals, platinum is hypoallergenic which makes it perfect for use in medical devices and implants. A platinum coating may have a protective role to prevent bacterial growth and/or corrosion of the implant. It may also be used to improve the structural integrity of the implant seeing that platinum is an exceptionally hard and durable metal. Such a protective coating thus extends the lifetime of the implant, as much as it does for medical tools such as clamps and scalpels. Arterial catheters and coronary stents contain platinum markers, given the radio-opacity of the metal, enabling doctors to guide and position the device during the treatment.
3. Catalytic performance
Except for its intrinsic physical properties, platinum is also well-known for its ability to absorb hydrogen, which found its applications in the chemical and automotive industries. Platinum absorbs excess hydrogen which improves catalytic performance.
- In the car engine, platinum acts as an integral part of a catalytic converter, facilitating the complete combustion of unburned hydrocarbons, carbon monoxide, and particulate matter passing through the exhaust into carbon dioxide and water.
- In the chemical industry, platinum is used as a catalyst for the production of many important chemicals, such as nitric acid, paraxylene (an intermediate in the production of PET), specialty silicones, and benzene.
- For many decades, platinum has also been used for so-called catalytic reforming, that is, reforming or breaking down petroleum into high-octane liquid products (blending stock) which are later used for the production of industrial chemicals.
4. Aerospace
Gas turbine jet engines in aircraft produce a lot of heat, with the operating temperature being 1500°C or higher. Turbine blades thus must be designed to withstand these extreme conditions, which is achieved with internal air cooling and protective coatings. As platinum has a really high melting point, it is commonly applied as a heat-resistant coating for engine parts and turbine blades, as well as for corrosion protection.
5. Corrosion protection
Because it is non-reactive with air, water, acid, or bases, platinum coatings can also be used for corrosion protection if other anti-corrosion options are not available.
6. Sensors
Platinum is well-known to have excellent sensitivity to gases and is used in a variety of sensing applications. It is routinely used to detect oxygen levels in industrial applications, biological systems, and car exhaust systems, among others. It is also used in home and industrial safety equipment for detecting carbon monoxide, nitrogen oxides, and other toxic gases in the air.
7. Fuel Cells
Fuel cells are a promising replacement for traditional battery technology used today. Fuel cells are electrochemical devices that convert the energy of a chemical reaction between hydrogen and oxygen directly into electricity, with heat and water as by-products. The role of platinum is to catalyze this reaction at room temperature as well as to catalyze the generation of protons needed for the reaction.
The fuel cell technology promises clean-energy mobility, efficient building power generators, and long-term energy storage.
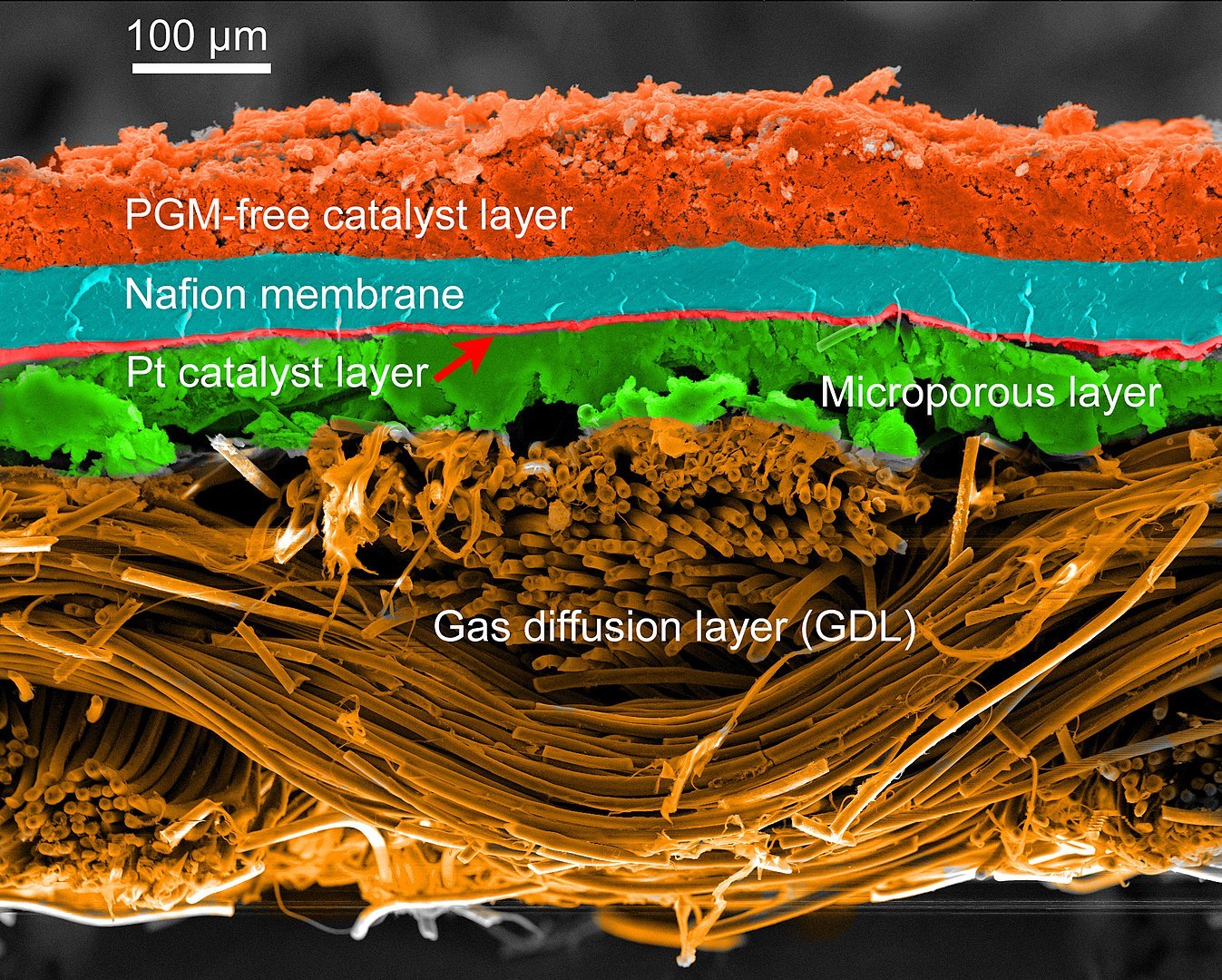 Thin platinum layer drives the catalytic reaction in fuel cells.
Thin platinum layer drives the catalytic reaction in fuel cells.
8. Computer hard disks
Platinum is used in computer hard disks within magnetic layers to improve data storage.
OrelTech has recently introduced a new product family based on platinum. The platinum product family consists of three products with unique metalized forms, each suitable for a specific application. To learn more about our platinum inks, visit our product site.
